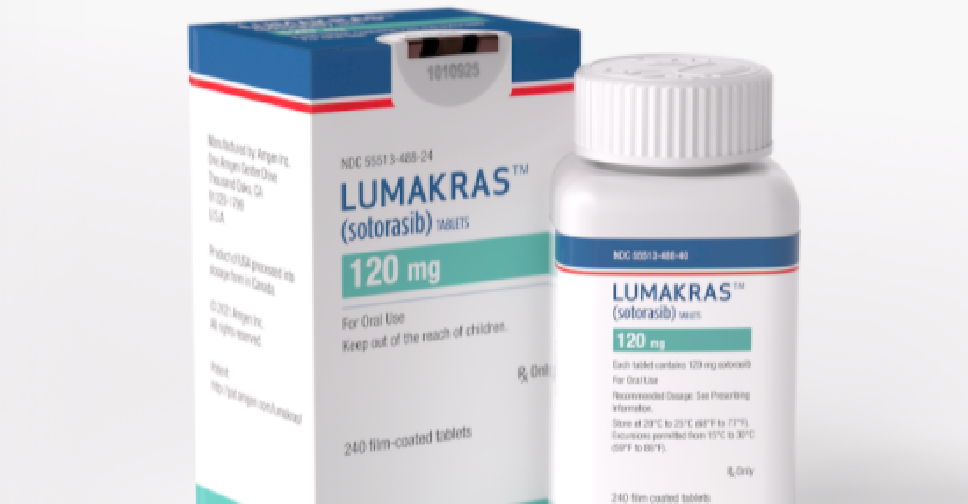
The UAE has become the second country in the world to approve the new lung cancer drug 'Lumakras'.
The Ministry of Health and Prevention (MoHAP) has cleared the registration and use of the drug, which recently received the approval of the US Food and Drug Administration (FDA).
It will help speed up the treatment plan of patients in the UAE and improve their quality of life.
Lumakras, manufactured by Amgen, is prescribed to adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), who have received at least one previous cancer therapy.
It is supplied as film-coated tablets for oral use containing 120 mg of sotorasib.




 UAE stresses diplomacy, security rights at GCC-EU meeting
UAE stresses diplomacy, security rights at GCC-EU meeting
 Etihad Airways to resume limited flight schedule
Etihad Airways to resume limited flight schedule
 H.H. Sheikh Hamdan, Saudi Defence Minister discuss regional developments
H.H. Sheikh Hamdan, Saudi Defence Minister discuss regional developments
 MOD send UAE residents missile and drone alert from Iran, Thursday evening
MOD send UAE residents missile and drone alert from Iran, Thursday evening
 Dubai strengthens smart monitoring, community reporting
Dubai strengthens smart monitoring, community reporting




