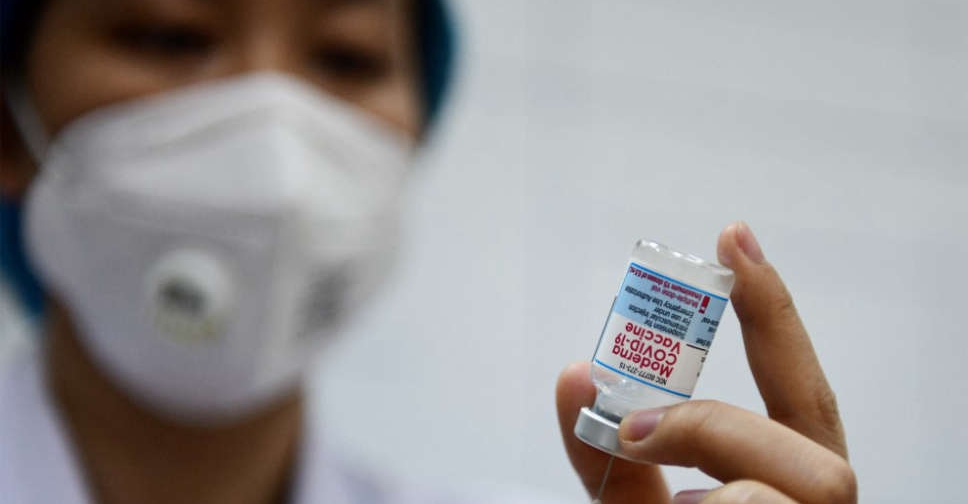
The US Food and Drug Administration on Monday gave full approval to Moderna's COVID-19 vaccine for people aged 18 and older, making it the second fully approved vaccine for the virus.
The Moderna vaccine has been authorised for emergency use in the United States since December 2020, and will now be sold under the brand name Spikevax.
Pfizer and BioNTech's COVID-19 shot using similar technology received full approval in the United States last year for people aged 16 and older after also first gaining emergency authorisation.
Nearly 75 million people have already received Moderna's two-dose vaccine in the United States, according to data from the US Centers for Disease Control and Prevention.
"The public can be assured that Spikevax meets the FDA’s high standards for safety, effectiveness and manufacturing quality required of any vaccine approved for use in the United States," Acting FDA Commissioner Dr. Janet Woodcock said in a statement.
Moderna's vaccine is cleared for use in more than 70 countries.



 Pope Francis meets prisoners and artists in first visit to Venice
Pope Francis meets prisoners and artists in first visit to Venice
 Harvey Weinstein hospitalised days after conviction overturned
Harvey Weinstein hospitalised days after conviction overturned
 Gold Titanic pocket watch sells for 4.1m dirham at auction
Gold Titanic pocket watch sells for 4.1m dirham at auction
 Arab ministerial coordination meeting reiterates ending Gaza conflict
Arab ministerial coordination meeting reiterates ending Gaza conflict
 Lebanon moves towards accepting ICC jurisdiction for war crimes on its soil
Lebanon moves towards accepting ICC jurisdiction for war crimes on its soil



