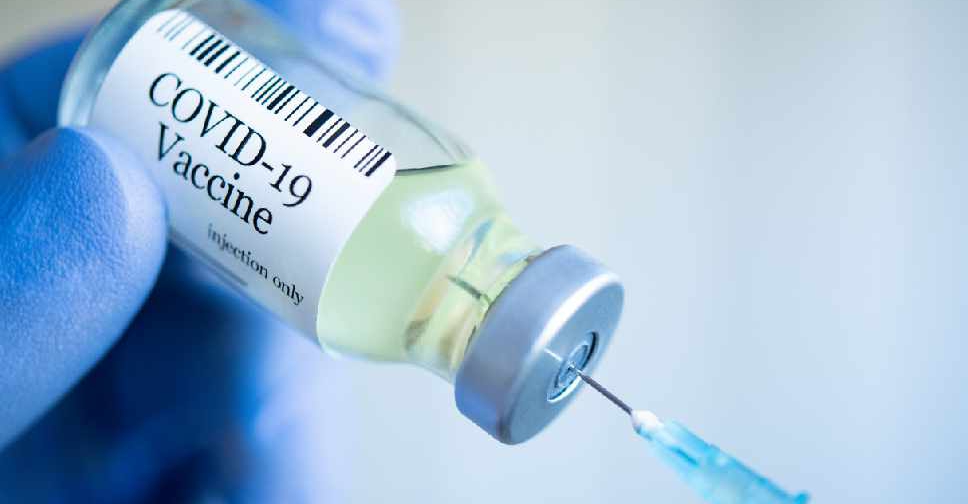
Sinovac Biotech said on Saturday that its unit's COVID-19 vaccine has been formally approved for use by the general public by China's medical products regulator.
It marks the second COVID-19 vaccine green-lighted for public use in China, after a shot developed by a Beijing institute affiliated to state-owned China National Pharmaceutical Group (Sinopharm) was approved in December.
Prior to the approvals, both vaccines have already been used in China's vaccination program mainly targeting key groups deemed to be at higher risk of exposure to the virus.
Indonesia, Turkey, Brazil, Chile, Colombia, Uruguay, and Laos have granted emergency authorizations for the CoronaVac vaccine developed by Sinovac Life Sciences, Sinovac said in a news release.
The approval is based on the two-month results from late-stage clinical trials overseas, from which the final analysis data has not yet been obtained, Sinovac said.




 US Epstein files release highlights Clinton, makes scant reference to Trump
US Epstein files release highlights Clinton, makes scant reference to Trump
 Bangladesh holds state funeral for slain youth leader amid tight security
Bangladesh holds state funeral for slain youth leader amid tight security
 US hits ISIS in Syria with large retaliatory strikes, officials say
US hits ISIS in Syria with large retaliatory strikes, officials say
 Pakistan court hands Imran Khan, wife 17-year jail terms in another graft case
Pakistan court hands Imran Khan, wife 17-year jail terms in another graft case
 Seven elephants killed in India train accident
Seven elephants killed in India train accident




