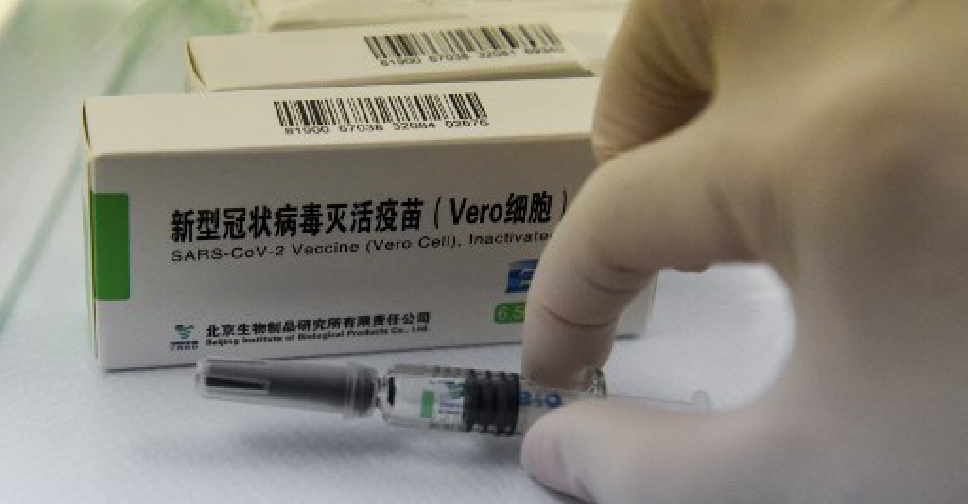
The World Health Organization announced on Friday it had approved a COVID-19 vaccine from China's state-owned drugmaker Sinopharm for emergency use.
The vaccine, one of two main Chinese shots that collectively have already been given to hundreds of millions of people in China and other countries including the UAE, becomes the first COVID-19 shot developed by a non-Western country to win the WHO's backing.
It is also the first time the WHO has given emergency use approval to any Chinese vaccine for any infectious disease.
WHO today listed the Sinopharm #COVID19 vaccine for emergency use in all countries, a prerequisite for a potential #COVAX roll-out. Interim policy recommendations were also issued for the vaccine usage.
— World Health Organization (WHO) (@WHO) May 7, 2021
👉 https://t.co/wuvNptP1LV pic.twitter.com/hm6TlpLJSy
In its approval, the WHO said Sinopharm's easy storage requirements make it highly suitable for low-resource settings, boosting supply to underserved countries.
It's also the first to carry a vaccine vial monitor, a small sticker on the vaccine vials that changes color as the vaccine is exposed to heat, letting health workers know whether if it can be safely used.
A WHO emergency listing is a signal to national regulators on a product's safety and efficacy, and would allow the shot to be included in COVAX, the global programme to provide vaccines mainly for poor countries.
The WHO has previously given emergency approval to COVID-19 vaccines developed by Pfizer-BioNTech, AstraZeneca, Johnson & Johnson, and, last week, Moderna.



 Earthquakes hit the Red Sea, near Saudi Arabia and Sudan
Earthquakes hit the Red Sea, near Saudi Arabia and Sudan
 Russia's Lavrov says US-South Korea nuclear guideline adds concern
Russia's Lavrov says US-South Korea nuclear guideline adds concern
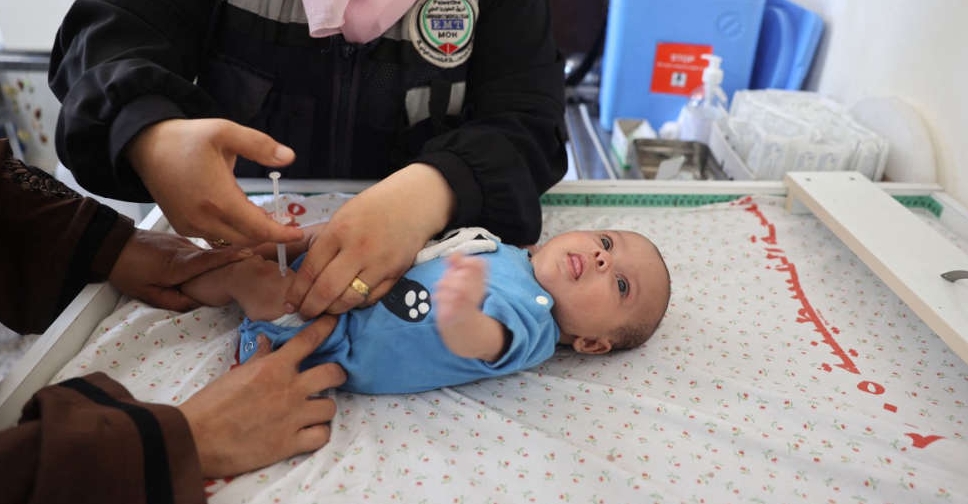 WHO to send one million polio vaccines to Gaza to protect children
WHO to send one million polio vaccines to Gaza to protect children
 'El Mayo', alleged Mexican kingpin, pleads not guilty to US charges
'El Mayo', alleged Mexican kingpin, pleads not guilty to US charges
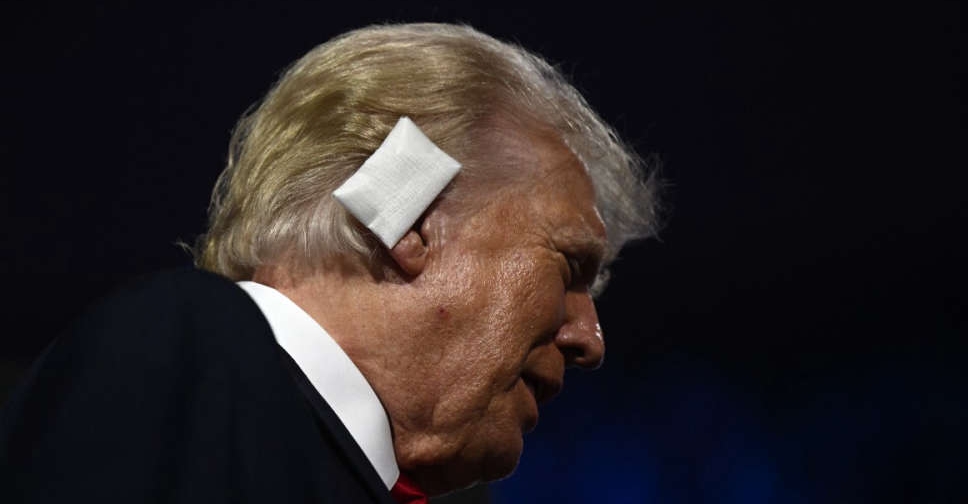 Trump doctor disputes suggestion that shrapnel, not bullet, hit his ear
Trump doctor disputes suggestion that shrapnel, not bullet, hit his ear




