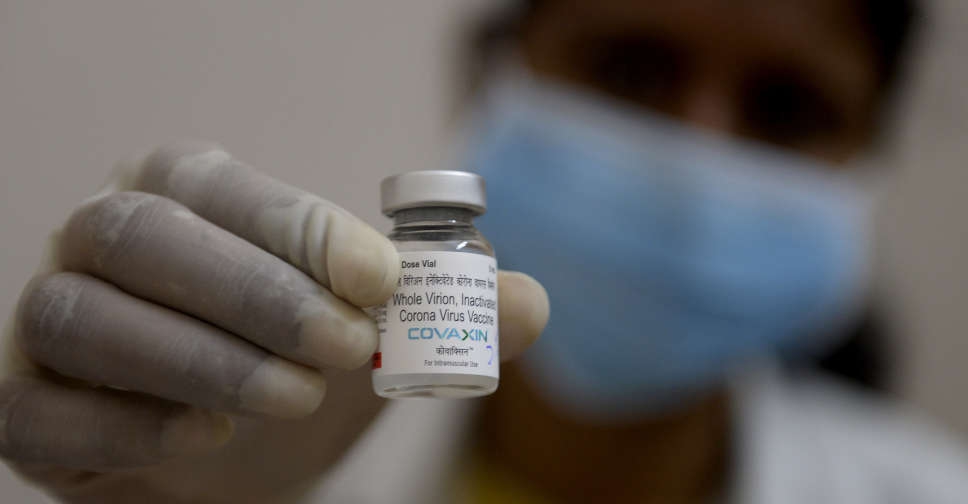
A World Health Organisation (WHO) technical advisory group was reviewing data on India's Covaxin shot against COVID-19 on Tuesday with a decision on its emergency use listing likely soon, a spokesperson said.
"If all is in place and all goes well and if the committee is satisfied, we would expect a recommendation within the next 24 hours or so," Margaret Harris told journalists at a UN press briefing.
Millions of Indians have taken the shot produced by Bharat Biotech but many have been unable to travel pending the WHO approval.




 No evidence alleged Bondi gunmen received military training in Philippines
No evidence alleged Bondi gunmen received military training in Philippines
 At least 12 killed in Nigeria mining site attack
At least 12 killed in Nigeria mining site attack
 Russian attack on Ukraine's central Cherkasy injures six, causes blackouts
Russian attack on Ukraine's central Cherkasy injures six, causes blackouts
 UN, aid groups warn Gaza operations at risk from Israel impediments
UN, aid groups warn Gaza operations at risk from Israel impediments
 Israel approves natural gas deal with Egypt, Netanyahu says
Israel approves natural gas deal with Egypt, Netanyahu says




